.
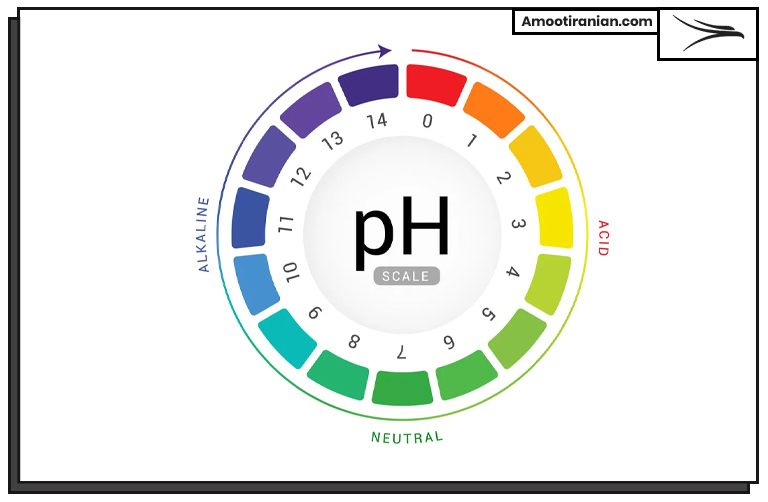
PH is a measure of the acidity or alkalinity of a solution. Urea solutions have a slightly acidic PH, ranging from around 5 to 6.5.
.
What is PH?
.

.
.
PH is a measure of the acidity or alkalinity of a solution.
.
It is defined as the negative logarithm of the concentration of hydrogen ions (H+) in a solution. In other words, PH is a measure of how many hydrogen ions are present in a solution.
The PH scale ranges from 0 to 14, with a PH of 7 considered neutral. Solutions with a PH below 7 are acidic, while solutions with a PH above 7 are basic or alkaline. The lower the PH value, the more acidic the solution is, and the higher the PH value, the more basic or alkaline the solution is.

.
The PH of a solution can affect many chemical and biological processes, such as:
- enzyme activity
- microbial growth
- chemical reactions
.
In living organisms, maintaining a proper PH level is important for many PHysiological processes, such as blood PH, which is tightly regulated to ensure proper functioning of the body’s organs and systems.
PH can be measured using a PH meter, PH paper or indicator, or other chemical tests.
It’s important to monitor PH levels in certain applications, such as in soil for agriculture or in water treatment, to ensure optimal conditions for growth or treatment.
.
What Is Urea PH?
.

.
Urea PH refers to the acidity or basicity level of a solution containing urea, a nitrogen-containing compound often used as a fertilizer.
.
The PH of urea solutions can vary depending on factors such as:
- the concentration of urea
- the presence of other chemicals or impurities
- the temperature of the solution
.
Is urea an acid or alkaline?
In general, urea solutions have a slightly acidic PH, ranging from around 5 to 6.5. This is because urea molecules can react with water to form ammonium and bicarbonate ions, which can increase the acidity of the solution. However, the PH of urea solutions can also be affected by other factors, such as the presence of other chemicals that can alter the acidity or basicity of the solution.
It is important to monitor the PH of urea solutions, as solutions with extremely low or high PH levels can lead to reduced fertilizer efficiency or even plant damage.
.
In general, a PH of around 6 is considered optimal for urea solutions used in agricultural applications, as this level is most conducive to the uptake of nutrients by plants. PH levels outside of this range can lead to nutrient loss or other problems that can affect plant growth and yield.
.
Why Does Urea Increase PH of the Soil?
How does urea affect the soil? Does urea increase PH? How does urea fertilizer affect pH? How does fertilizer increase soil pH?
Urea is a nitrogen-containing compound that is commonly used as a fertilizer in agriculture.
When urea is applied to the soil, it can undergo a chemical reaction called hydrolysis, which releases ammonium ions (NH4+) and bicarbonate ions (HCO3-) into the soil.
The ammonium ions produced by hydrolysis are positively charged, and they can displace positively charged hydrogen ions (H+) in the soil, which can lead to an increase in soil PH.
This is because the presence of more ammonium ions in the soil means there are fewer hydrogen ions available to lower the PH of the soil.
In addition, the bicarbonate ions produced by hydrolysis can act as a buffer, helping to maintain a stable PH level in the soil. Bicarbonate ions are able to absorb or release hydrogen ions depending on the acidity or alkalinity of the soil, which can help to prevent drastic changes in PH.
.
It’s important to note that the effects of urea on soil PH can vary depending on factors such as the type of soil, the concentration of urea applied, and the timing and frequency of application.
It’s also important to monitor soil PH levels to ensure that they remain within the optimal range for plant growth, as excessively high or low PH levels can lead to reduced plant growth and nutrient uptake.
.
What Fertilizer Raises PH & What Fertilizer Lowers It?
| Fertilizer | Effect on Soil PH |
| Agricultural lime | Raises soil PH levels |
| Wood ash | Raises soil PH levels |
| Bone meal | Raises soil PH levels |
| Dolomite lime | Raises soil PH levels |
| Marl | Raises soil PH levels |
| Potassium hydroxide | Raises soil PH levels |
| Calcium carbonate | Raises soil PH levels |
| Sodium nitrate | Lowers soil PH levels |
| Ammonium nitrate | Lowers soil PH levels |
| Sulfur | Lowers soil PH levels |
| Iron sulfate | Lowers soil PH levels |
| Urea | Raises soil PH levels |
.
As mentioned earlier, the effect of urea on soil PH can vary depending on various factors such as soil type, amount and frequency of application, and other environmental factors.
.
What PH Is Best for Soil?
.
The optimal PH for soil depends on the types of plants or crops that are being grown. However, in general, most plants prefer a slightly acidic soil with a PH between 6.0 and 7.0.
.
When the soil PH is too low (acidic), nutrients such as calcium, magnesium, and PHosPHorus become less available to plants, which can result in stunted growth and other nutrient deficiencies.
When the soil PH is too high (alkaline), micronutrients such as iron, manganese, and zinc may become less available, which can also result in reduced growth and other problems.
.
Soil PH can be tested using a soil PH meter, PH test kits, or by sending a soil sample to a laboratory for analysis.
.
If the soil PH is too high or too low for the specific plants or crops being grown, adjustments can be made by adding soil amendments such as lime to raise the PH or sulfur to lower the PH.
It’s important to note that soil PH is just one factor that affects plant growth, and other factors such as soil structure, nutrient availability, and water availability should also be considered when trying to optimize soil conditions for plant growth.
.

Writer: Mohsen Yadegari
Sales Specialist at Amoot Iranian Company
