.
The petroleum industry is the highest consumer of sulfur, primarily for the production of sulfuric acid, which is used in a wide range of industrial applications.
.
.
.
What Is Sulfur Most Commonly Used in?
The petroleum industry is the biggest consumer of sulfur due to its extensive use in the production of sulfuric acid that finds applications across a range of industries.
Sulfur is also used as a component in the production of other chemicals such as fertilizers, detergents, and pesticides.
Additionally, sulfur is used in the metallurgical industry for the production of iron and steel, as well as in the production of paper and pulp.
However, the largest demand for sulfur is still from the petroleum industry, which accounts for over 70% of global sulfur consumption.
.
.
.
Why Most of the World’s Sulfur is Used for the Production of Sulfuric Acid?
.
The primary reason why most of the world's sulfur is used for the production of sulfuric acid is that sulfuric acid is a highly versatile and useful chemical that finds applications across a wide range of industries.
.
Sulfuric acid is a strong mineral acid that is widely used in industrial processes, including the production of fertilizers, chemicals, and metals. It is also used in the manufacture of a variety of consumer products, including detergents, batteries, and textiles.
Sulfuric acid is produced by reacting sulfur dioxide (SO2) with oxygen (O2) and water (H2O) in the presence of a catalyst. Sulfur dioxide is usually obtained from the burning of sulfur or from the roasting of metal sulfide ores.
The production of sulfuric acid is a large-scale industrial process that requires specialized equipment and infrastructure, which is why it is usually carried out by large chemical companies.
Sulfuric acid is a highly corrosive and dangerous substance that requires careful handling and storage.
.
.
.
However, its versatility and usefulness have made it an essential component of many industrial processes, which is why most of the world’s sulfur is used for its production.
.
Why Is Sulphuric Acid the King of Chemicals?
Sulfuric acid is often referred to as the “king of chemicals” due to its wide range of industrial applications and its importance to many industrial processes. Here are some reasons why sulfuric acid is considered the king of chemicals:
.
1_ Versatility: Sulfuric acid is a highly versatile chemical that finds applications across a wide range of industries, including fertilizers, chemicals, metals, and consumer products. It is also used in wastewater treatment and in the production of clean energy.
.
2_ Production volume: Sulfuric acid is one of the most widely produced chemicals in the world, with global production exceeding 200 million metric tons per year. This high production volume is a testament to its importance to many industrial processes.
.
3_ Strong acid: Sulfuric acid is a highly acidic substance that can dissolve many materials, including metals, rocks, and organic matter. This property makes it useful for many industrial processes, such as metal refining and the production of detergents and other cleaning products.
.
4_ Dehydrating agent: Sulfuric acid is a powerful dehydrating agent, which means that it can remove water from many substances. This property makes it useful in the production of many chemicals, such as nitric acid and hydrochloric acid.
.
Overall, sulfuric acid’s versatility, high production volume, strong acidity, and dehydrating properties make it an essential chemical for many industrial processes, which is why it is considered the king of chemicals.
.
How Much Sulfur Is in Sulfuric Acid?
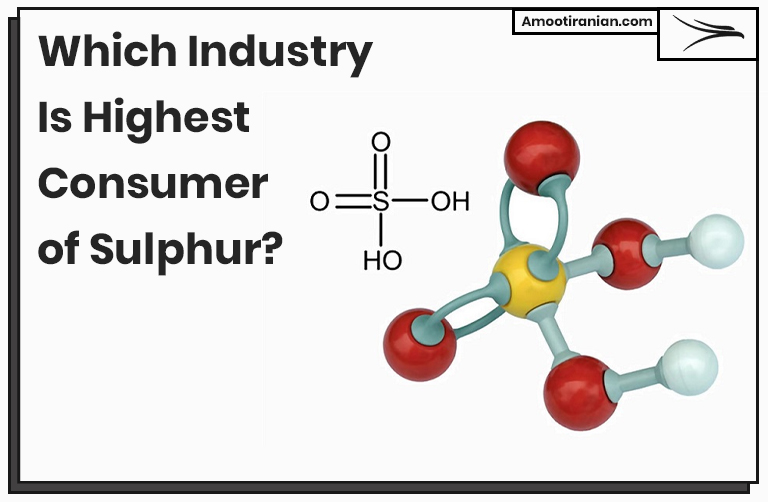
Sulfuric acid has the chemical formula H2SO4, which means that it contains two hydrogen atoms, one sulfur atom, and four oxygen atoms. The atomic mass of sulfur is 32.065 g/mol, while the atomic mass of oxygen is 15.999 g/mol.
.
Therefore, the molar mass of sulfuric acid is:
2(1.008 g/mol) + 32.065 g/mol + 4(15.999 g/mol) = 98.079 g/mol
.
This means that sulfur makes up 32.65% of the molar mass of sulfuric acid, which is calculated as:
(32.065 g/mol / 98.079 g/mol) x 100% = 32.65%
.
So, there is approximately 32.65% sulfur by mass in sulfuric acid.
.
Does 100% Sulfuric Acid Exist?
.
No, 100% sulfuric acid does not exist under normal conditions.
.
Sulfuric acid is a highly reactive and corrosive substance that can cause severe chemical burns and damage to organic materials.
It is also a powerful dehydrating agent, which means that it can remove water from many substances.
Pure sulfuric acid has a boiling point of around 337 °C (639 °F) and decomposes at temperatures above this point.
However, the boiling point of sulfuric acid varies depending on its concentration. The most common concentration of commercial sulfuric acid is 98%, which means that it contains 98% sulfuric acid and 2% water by mass.
Obtaining sulfuric acid with a concentration of 100% is not possible using conventional distillation methods due to its tendency to decompose at high temperatures.
However, it is possible to obtain a highly concentrated form of sulfuric acid called oleum (also known as fuming sulfuric acid), which is a mixture of sulfuric acid and sulfur trioxide.
Oleum can have a sulfuric acid concentration of up to 115%, but it is highly reactive and requires specialized handling and storage.
.

.
.
What Will Happen to the World without Sulfuric Acid?
.
Without sulfuric acid, many industrial processes would be severely impacted, as sulfuric acid is a crucial component of many chemical reactions.
.
For example, sulfuric acid is used to produce fertilizers, which are essential for modern agriculture. It is also used in the production of metals, such as copper and zinc, and is a key component of many industrial chemicals, including dyes, pigments, and explosives.
In addition, sulfuric acid is also used in the manufacture of a wide range of consumer products, including batteries, detergents, and textiles.
Without sulfuric acid, the production of these products would be significantly curtailed or may require the development of new chemical processes.
.

.
Furthermore, sulfuric acid is also used in wastewater treatment and in the production of clean energy.
In wastewater treatment, sulfuric acid is used to adjust the pH of the water to prevent the growth of harmful microorganisms.
In the production of clean energy, sulfuric acid is used in the manufacture of photovoltaic cells for solar panels.
In short, without sulfuric acid, many industrial processes would be severely impacted, and it would be challenging to maintain the same level of technological and economic development that we currently enjoy.
.
Which Industries Use Sulfur?
The table below shows the entire industries that use sulfur chemical.
.
| Industry | Use of Sulfur |
| Petroleum | Production of sulfuric acid for various applications |
| Fertilizer | Production of sulfuric acid for manufacturing fertilizers |
| Chemical | Production of sulfuric acid, sulfates, and sulfur dioxide |
| Metallurgical | Production of iron and steel, and purification of copper and zinc ores |
| Paper and Pulp | Bleaching agent, removal of lignin from wood pulp |
| Food | Preservative, prevention of microbial growth in food products |
| Rubber | Cross-linking of rubber molecules for improved strength and durability |
| Textile | Production of dyes and removal of impurities from fabrics |
| Water Treatment | Removal of impurities and neutralization of acidic water |
.

.
Here is some additional information on each industry:
1_Petroleum: The petroleum industry is the largest consumer of sulfur, and it is primarily used in the production of sulfuric acid, which has a wide range of applications in various industries. Sulfuric acid is used for oil refining, metal processing, fertilizer manufacturing, and many other industrial processes.
.
2_Fertilizer: Sulfuric acid is a key component in the manufacturing of fertilizers, which are used to promote plant growth and increase crop yields. Fertilizers such as ammonium sulfate and superphosphate are made by reacting sulfuric acid with other chemicals.
.
3_Chemical: Sulfur is used in the production of a wide range of chemicals, including sulfuric acid, sulfates, and sulfur dioxide. These chemicals are used in the production of fertilizers, detergents, pesticides, and many other products.
.
4_Metallurgical: Sulfur is used in the metallurgical industry for the production of iron and steel. It is also used in the purification of copper and zinc ores, where it reacts with impurities to form sulfur dioxide, which can be removed through a process called smelting.
.
5_Paper and Pulp: Sulfur is used in the paper and pulp industry as a bleaching agent and to remove lignin from wood pulp. Lignin is a natural polymer that gives wood its strength, but it also makes it difficult to process into paper.
.
6_Food: Sulfur dioxide is used as a preservative in the food industry to prevent microbial growth and extend the shelf life of food products. It is commonly used in dried fruits, wine, and other products.
.
7_Rubber: Sulfur is used in the production of rubber to improve its strength and durability. When heated, sulfur reacts with rubber molecules to form cross-links, which give the rubber its characteristic properties.
.
8_Textile: Sulfur is used in the production of dyes and to remove impurities from fabrics. It is used in the textile industry to make cotton and other fibers more receptive to dyes and to remove unwanted impurities.
.
9_Water Treatment: Sulfur is used in the water treatment industry to remove impurities and neutralize acidic water. Sulfur can react with impurities such as iron and manganese to form insoluble compounds that can be removed through filtration.
