.
.
.
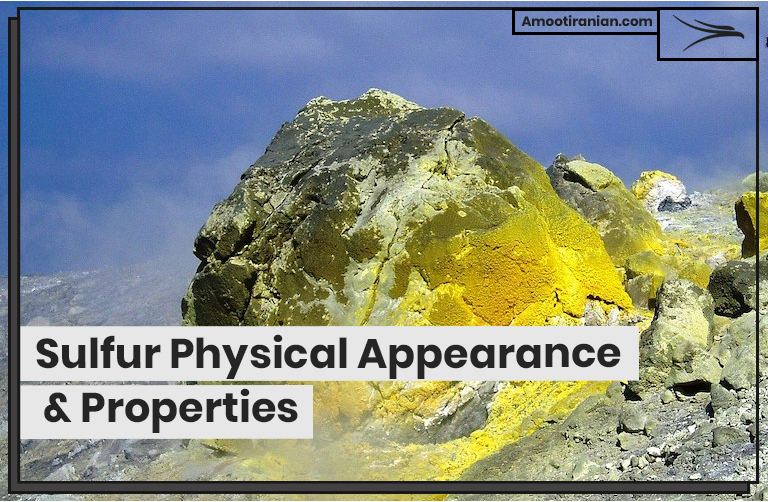
| Appearance | Sulfur is a yellow, crystalline solid at room temperature. |
| Odor | Sulfur has a distinctive, strong odor that is often described as being similar to rotten eggs. |
| Melting point | The melting point of sulfur is 115.21 °C (239.38 °F). |
| Boiling point | The boiling point of sulfur is 444.6 °C (832.3 °F). |
| Density | The density of sulfur is 2.07 g/cm3. |
| Solubility | Sulfur is insoluble in water, but it is soluble in organic solvents like carbon disulfide and benzene. |
| State of matter | Sulfur is a solid at room temperature and pressure, but it can be melted or vaporized under certain conditions. |
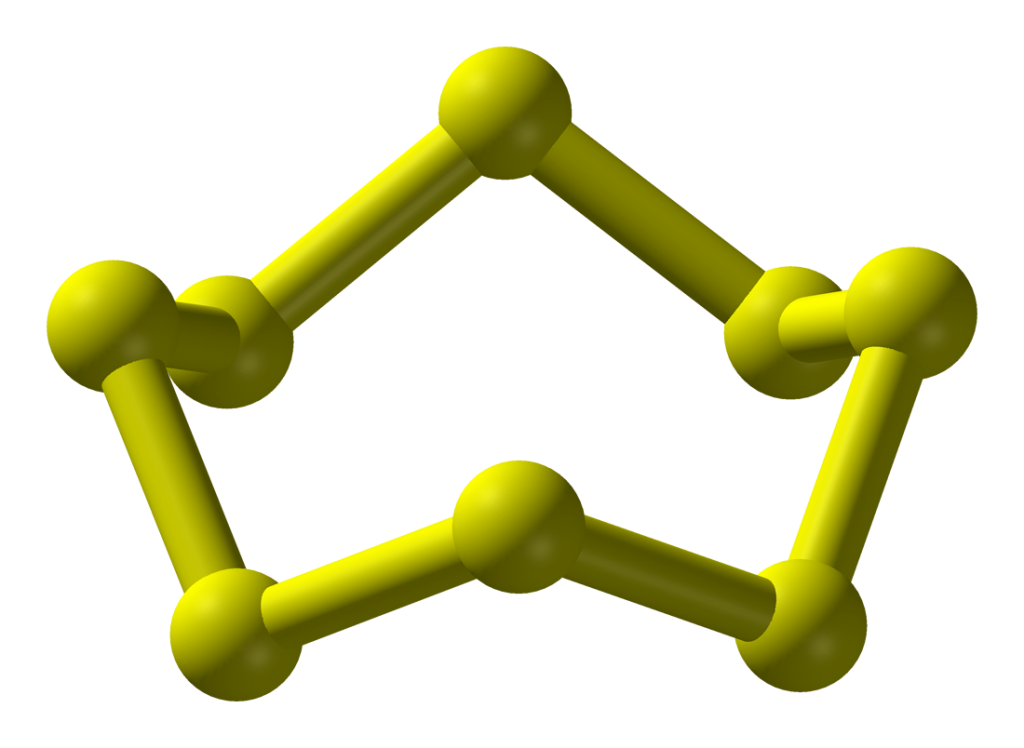
| Crystal structure | Sulfur has a rhombic crystal structure at room temperature, but it can also exist in other crystal structures at different temperatures and pressures. |
| Hardness | Sulfur has a hardness of 1.5 on the Mohs scale, which means it is a relatively soft mineral. |
| Conductivity | Sulfur is a poor conductor of electricity and heat. |
| Color | Sulfur is typically yellow in color, but it can also appear in shades of brown, red, or black depending on the impurities present. |
| Molecular weight | The molecular weight of sulfur is 32.06 g/mol. |
| Viscosity | Sulfur has a low viscosity, which means it flows relatively easily. |
| Flammability | Sulfur is flammable and can ignite when exposed to a flame or spark. |
| Reactivity | Sulfur can react with many other elements and compounds, including metals, acids, and halogens. |
| Toxicity | Sulfur is not highly toxic, but it can cause irritation or damage to the skin, eyes, and respiratory system if ingested or inhaled in large quantities. |
| Magnetic properties | Sulfur is diamagnetic, which means it is not attracted to a magnet. |
| Thermal conductivity | Sulfur has a low thermal conductivity, which means it does not transfer heat well. |
| Stability | Sulfur is a relatively stable element under normal conditions, but it can undergo oxidation or reduction reactions in certain environments. |
| Optical properties | Sulfur is opaque to visible light and has a relatively low refractive index. |
| Radioactivity | Sulfur has no radioactive isotopes, so it is not radioactive. |
| Corrosivity | Sulfur can corrode certain metals, especially in the presence of water or other corrosive agents. |
| Hygroscopicity | Sulfur can absorb moisture from the air and become damp or sticky. |
| Tensile strength | Sulfur is relatively brittle and has a low tensile strength, meaning it is not well-suited for use in structural applications. |
.
It’s worth noting that some of these properties can vary depending on the specific form of sulfur and the conditions under which it is present.
.
For example, sulfur can exist in different allotropes with different physical properties, and its melting and boiling points can change depending on the pressure and atmospheric conditions.
