Sulfur is a chemical element with the symbol “S” and atomic number 16. It is a non-metal that has a yellow color in its solid form and is a gas at room temperature.
Three different ways of sulfur production are explained fully in this article including Frasch, Claus, and Sicilian methods.
.
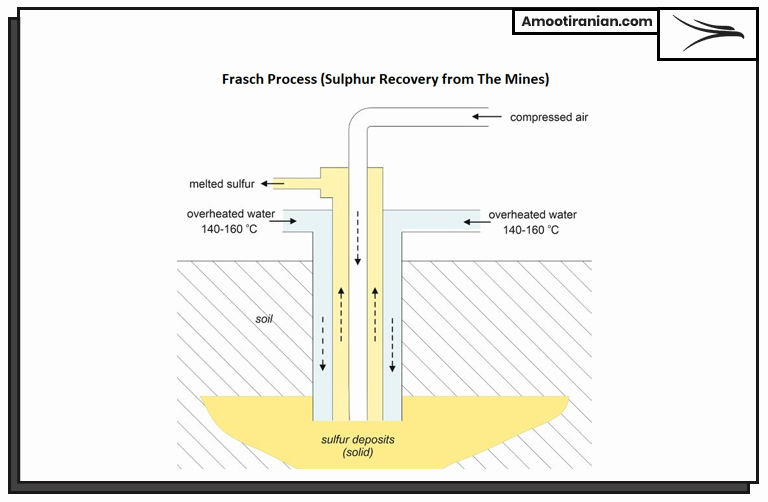
1_Frasch Process (Sulphur Recovery from The Mines)
This process involves the mining of sulfur through the use of wells drilled into sulfur deposits, followed by melting the sulfur underground using superheated water and pumping the liquid sulfur to the surface.
The sulfur is then cooled and solidified for further processing and use.
.
.
Frasch process includes the following steps:
First: Drilling
Wells are drilled into the sulfur deposit, which can be located hundreds or even thousands of meters underground.
Second: Superheating
Superheated water is pumped into the wells, which melts the sulfur and forms a hot, liquid sulfur pool underground.
Third: Extraction
A hollow tube called a “mule” is lowered into the liquid sulfur pool. The sulfur is forced up the tube by high-pressure steam, which creates a cavity around the tube.
Forth: Pumping
As the tube is raised to the surface, the sulfur is pumped out of the cavity and into a collection tank.
Fifth: Solidification
Once the sulfur has been collected, it is cooled and solidified, typically in a “block” form for ease of transportation and storage.
Sixth: Processing
The solid sulfur blocks can be further processed into a variety of forms, such as granules or powder, depending on the intended use.
Overall, the Frasch process is a relatively complex and expensive method of sulfur production, but it can be highly effective for extracting sulfur from deep underground deposits where other methods are not feasible.
.
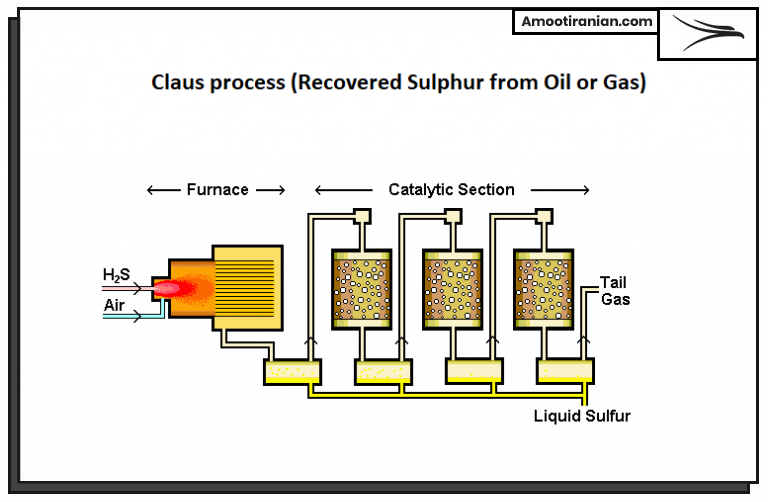
2_Claus process (Recovered Sulphur from Oil or Gas)
This process involves the extraction of sulfur from the oil or gas stream at a processing plant. Hydrogen sulfide gas (H2S) is separated from the natural gas or crude oil and then processed in a series of reactions to produce elemental sulfur. The Claus process is the most commonly used method of sulfur production worldwide.
.
The refinery sulfur or Claus process has two main types:
- Gaseous Claus (GC): extract sulfur from gas
- Petroleum Claus (OC): extract sulfur from oil
Overall, the Claus process is a widely used and efficient method of sulfur production, particularly in the petroleum industry where sour gas is a common byproduct. The process can be modified to recover even more sulfur from the gas stream, resulting in higher overall sulfur recovery rates.
Gaseous Claus type is used to extract sulfur from sour natural gas or other gas streams that contain H2S as an impurity. The gas is first treated to remove any contaminants, and then processed using the steps outlined in the previous answer.
Petroleum Claus type is used to extract sulfur from crude oil or other petroleum products that contain H2S. The H2S is first stripped from the oil using a variety of techniques, such as amine gas treating, and then processed using the same steps as in the gaseous Claus process.
.
3_Sicilian method
This method involves scraping sulfur from the surface of the earth or digging it out of open pits.
This method was commonly used in the past, particularly in the volcanic regions of Sicily, but it is now less common due to the depletion of easily accessible sulfur deposits.
Sicilian method in sulfur production includes the following steps:
First: Identification
The first step in the Sicilian method involves identifying areas where sulfur deposits may be located. In the past, this was often done by looking for areas with distinctive yellow or orange-colored soil, which can indicate the presence of sulfur.
Second: Excavation
Once a suitable deposit has been identified, the next step is to excavate the sulfur from the ground. This can involve scraping the sulfur from the surface of the earth, or digging it out of open pits or tunnels.
Third: Transportation
Once the sulfur has been extracted, it is transported to a processing facility or storage site. In some cases, the sulfur may be transported over long distances by ship or other means.
Forth: Processing
The extracted sulfur must then be processed to remove impurities and bring it to the desired level of purity. This can involve melting the sulfur and separating it from any other materials or impurities, as well as filtering or otherwise treating the sulfur to remove any contaminants.
Fifth: Storage and Transportation
The final step in the Sicilian method involves storing the processed sulfur and transporting it to its intended destination, where it can be further processed or used directly as a raw material.
