.

.
What Is The Process Of Producing Sulfuric Acid?
.
How do you make sulfuric acid step by step?
.
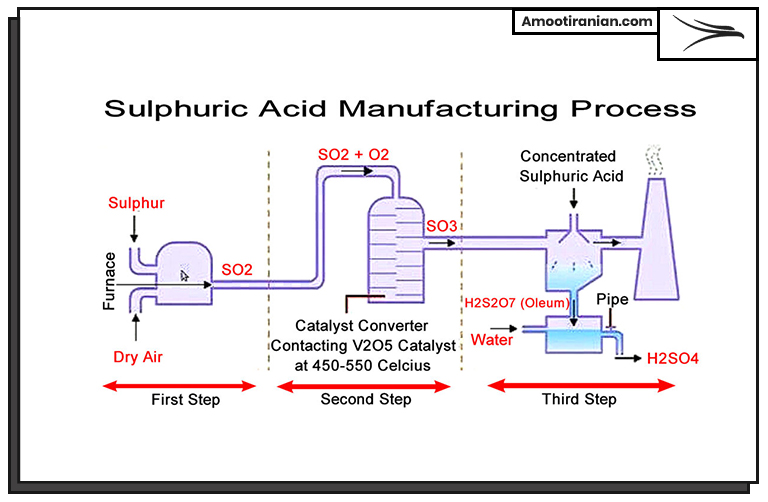
1. Sulfur Extraction
The process begins with the extraction of sulfur from natural sources, such as underground deposits or oil and gas refining processes.
Sulfur can also be obtained as a byproduct from metal smelting or natural gas processing.
.
.
2. Sulfur Combustion
The extracted sulfur is combusted in the presence of excess air to convert it into sulfur dioxide (SO2).
The combustion may take place in a dedicated sulfur burner or a furnace.
.
3. Sulfur Dioxide Conversion
The generated sulfur dioxide is then converted into sulfur trioxide (SO3) through a catalytic reaction.
.
.
Traditionally, the contact process is used, which involves passing the SO2 over a vanadium(V) oxide catalyst at a high temperature of around 450-500°C.
.
4. Absorption
The produced SO3 is mixed with concentrated sulfuric acid (H2SO4) in an absorption tower.
The tower typically contains a series of packed beds or trays to facilitate the contact between the gases and the liquid.
.
5. Sulfuric Acid Formation
The SO3 reacts with the H2SO4 in the absorption tower, forming oleum (a solution of sulfuric acid in sulfur trioxide) or pyrosulfuric acid (H2S2O7).
These intermediate products are highly corrosive and have a high concentration of sulfuric acid.
.
6. Dilution
If commercial-grade sulfuric acid is desired, the oleum or pyrosulfuric acid is diluted with water to the desired concentration.
The dilution is carefully controlled to prevent excessive heat generation and ensure safety.
.
7. Cooling
The diluted sulfuric acid is then cooled to reduce its temperature.
This step is important to avoid damage to equipment and facilitate safe handling of the acid.
.
8. Filtration
In some cases, the cooled sulfuric acid may be subjected to filtration to remove any impurities or solid particles that might be present.
This ensures the final product meets the desired purity standards.
.
“Sulfuric Acid Purification Process”
.
9. Storage and Packaging
The produced sulfuric acid is transferred to storage tanks made of materials resistant to corrosion, such as glass-lined steel or high-density polyethylene (HDPE).
.

.
From there, it can be packaged in various containers, such as drums, totes, or tankers, depending on the intended use and market needs.
.
10. Quality Control
Throughout the production process, samples of the sulfuric acid are collected and analyzed to ensure it meets the required specifications, such as concentration, impurity levels, and acidity.
.
It’s important to note that the sulfuric acid production process described here is a generalized overview.
Specific details and variations may exist depending on the plant’s design, scale, and technological advancements utilized.
Additionally, safety precautions and environmental considerations play a crucial role in the overall process to ensure the well-being of workers and minimize any negative impact on the environment.
.
.
Which Catalyst Is Best For Sulphuric Acid?
.
What Is The Catalyst Needed In The Contact Process Used In Producing Sulphuric Acid Industrially?
The catalyst used in the contact process for producing sulfuric acid industrially is vanadium(V) oxide (V2O5).
.
It acts as a catalyst to facilitate the conversion of sulfur dioxide (SO2) to sulfur trioxide (SO3) in the presence of oxygen.
The vanadium(V) oxide catalyst enhances the reaction rate and improves the overall efficiency of the process.
It is typically supported on a substrate, such as silica or alumina, to increase its surface area and enhance contact between the reactants.
.
.
Sulfuric Acid Production Reactions
The production of sulfuric acid involves a series of reactions.
Here are the main reactions involved in the contact process:
.
1. Sulfur Combustion
S(s) + O2(g) → SO2(g) Sulfur (S) is combusted in the presence of oxygen (O2) to produce sulfur dioxide (SO2) gas.
.
2. Sulfur Dioxide Conversion
2SO2(g) + O2(g) ⇌ 2SO3(g) In the presence of a catalyst (typically vanadium(V) oxide), sulfur dioxide (SO2) reacts with oxygen (O2) to form sulfur trioxide (SO3) gas. This reaction is reversible, and the forward reaction is favored at high temperatures.
.
3. Absorption
SO3(g) + H2SO4(l) → H2S2O7(l) The produced sulfur trioxide (SO3) gas is mixed with concentrated sulfuric acid (H2SO4) to form oleum (pyrosulfuric acid, H2S2O7). Oleum is a solution of sulfuric acid in sulfur trioxide and is a highly corrosive intermediate product.
.
4. Dilution
H2S2O7(l) + H2O(l) → 2H2SO4(l) Oleum (H2S2O7) is diluted with water (H2O) to produce the final product, sulfuric acid (H2SO4).
.
These reactions outline the main steps in the production of sulfuric acid through the contact process.
However, it’s important to note that industrial processes may involve additional steps, variations, or modifications to optimize efficiency and meet specific requirements.
