.

.
The density of sulfuric acid varies with concentration, but the most commonly used concentration of sulfuric acid is 98%, which has a density of about 1.84 grams per cubic centimeter (g/cm³) at room temperature (25°C or 77°F).
.
What Is the Density of Sulfuric Acid?
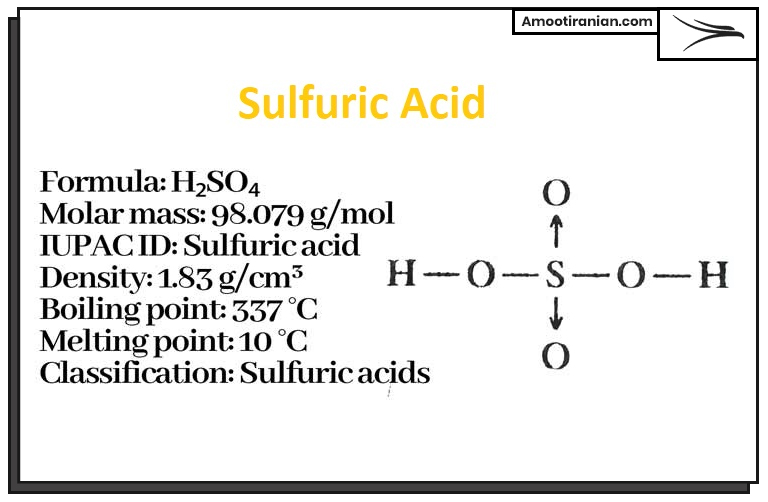
Sulfuric acid is a strong, highly corrosive, and dense liquid that is widely used in industrial processes such as the production of fertilizers, dyes, detergents, and various chemicals.
.
Its chemical formula is H2SO4, and it consists of hydrogen, sulfur, and oxygen atoms. Sulfuric acid is also used in laboratory experiments, battery production, and as a catalyst in many chemical reactions.
.
.
It is important to handle sulfuric acid with care as it is highly reactive and can cause severe burns or other hazards if not used properly.
.
What is the density of 98% H2SO4? What is the density of 70% H2SO4?
What is the density of 85% H2SO4? What is the density of 75% H2SO4?
The density of sulfuric acid varies depending on its concentration. Here are some typical values at room temperature (25°C or 77°F):
- 10% sulfuric acid: 1.07 g/cm³
- 25% sulfuric acid: 1.14 g/cm³
- 50% sulfuric acid: 1.39 g/cm³
- 75% sulfuric acid: 1.63 g/cm³
- 98% sulfuric acid: 1.84 g/cm³
.
It’s important to note that sulfuric acid is a highly corrosive and dangerous chemical. Always handle it with care and follow proper safety procedures.
.
Sulfuric Acid Density vs Concentration
The density of sulfuric acid changes with its concentration. Generally, the density of sulfuric acid increases with increasing concentration.
.
Here are some typical values of sulfuric acid density at room temperature (25°C or 77°F) for various concentrations:
- 10% sulfuric acid: 1.07 g/cm³
- 50% sulfuric acid: 1.39 g/cm³
- 98% sulfuric acid: 1.84 g/cm³
.
As shown, the density of sulfuric acid increases from around 1.07 g/cm³ for 10% concentration to 1.84 g/cm³ for 98% concentration.
.
How to Determine Sulfuric Acid Concentration?
.
There are several ways to determine the concentration of sulfuric acid in a solution, some of which are:
.
Methods to Determine Sulfuric Acid Concentration
| Method | Principle |
| Titration | Adding a known amount of base to the acid to neutralize it |
| Density | Measuring the density of the acid and comparing to a table |
| Conductivity | Measuring the electrical conductivity of the acid |
| Refractometry | Measuring the refractive index of the acid |
| Spectroscopy | Using light absorption or emission to determine the amount |
| pH measurement | Measuring the pH of the acid and comparing to a table |
.
Each of these methods has its own advantages and disadvantages, and the choice of method depends on the specific application and available equipment.
Titration is a common and widely used method, while density measurement is a quick and easy way to estimate the concentration.
Conductivity and refractometry are less common, but can be useful in certain situations. Spectroscopy is a highly accurate but more complex method, and pH measurement can provide a rough estimate of the concentration.
.
How to Determine Sulfuric Acid Density?
.
There are several methods for determining the density of sulfuric acid, including:
.
Different methods used to determine the density of sulfuric acid
| Method | Principle |
| Hydrometer | Measuring the buoyancy of the acid |
| Pycnometer | Measuring the mass and volume of a known amount of acid |
| Digital densitometer | Measuring the mass and volume using a digital instrument |
| Refractometry | Measuring the refractive index of the acid |
.
Each method has its own advantages and disadvantages, which are explained below.
.
The hydrometer is a simple and inexpensive method, but it requires calibration for accurate results.
The pycnometer is more accurate than the hydrometer, but it can be time-consuming and requires careful handling.
A digital densitometer is a more modern instrument that provides quick and accurate results. Refractometry is a less common method, but it can be useful in certain situations.
The choice of method depends on the specific application, available equipment, and level of accuracy required.
.
Advantages & Disadvantages of different methods used to determine sulfuric acid density
.
| Method | Principle | Advantages | Disadvantages |
| Hydrometer | Measuring the buoyancy of the acid | Simple, inexpensive, and portable. Does not require much equipment or technical knowledge. | Calibration is necessary for accurate results. Not very accurate for very concentrated solutions. |
| Pycnometer | Measuring the mass and volume of a known amount of acid | More accurate than hydrometer. Can be used for a wide range of concentrations. | Time-consuming, requires careful handling, and may introduce errors due to temperature changes. |
| Digital densitometer | Measuring the mass and volume using a digital instrument | Quick and accurate results. Can be used for a wide range of concentrations. | Expensive and requires technical knowledge. |
| Refractometry | Measuring the refractive index of the acid | Non-destructive and requires a very small sample size. Can be used for very concentrated solutions. | Limited accuracy for very dilute solutions. Not suitable for colored or opaque solutions. |
.
Is H2SO4 Denser than Water?
Yes, sulfuric acid (H2SO4) is denser than water.
.
The density of sulfuric acid varies with its concentration, but in general, its density is higher than that of water at room temperature.
For example, the density of 98% sulfuric acid is about 1.84 grams per cubic centimeter (g/cm³), while the density of water is about 1 gram per cubic centimeter (g/cm³) at the same temperature.
This means that sulfuric acid is about 1.8 times denser than water.
This difference in density between sulfuric acid and water is an important property that has several practical applications.
For example, it allows for the separation of these two substances in certain industrial processes that require the use of a dense liquid to form layers.
.
Additionally, it can be used to measure the concentration of sulfuric acid in a solution by determining its density using various instruments such as a hydrometer or a density meter.
However, it’s important to remember that sulfuric acid is highly corrosive and must be handled with care to prevent any accidents or harm to human health.
.
Is Sulfuric Acid Denser than Air?
Yes, sulfuric acid is denser than air. The density of sulfuric acid is around 1.84 grams per cubic centimeter (g/cm³) at room temperature and standard atmospheric pressure, while the density of air is around 0.0012 g/cm³.
.
This means that sulfuric acid is much more dense than air and will sink to the bottom if released into the air.
In addition to being heavier than air, sulfuric acid can also release toxic fumes when it comes into contact with water, which can pose a danger to human health and the environment.

great and helpful information
your website data usually answers my chemical sulfur questions