.

.
Sulfur trioxide is a chemical compound composed of three oxygen atoms and one sulfur atom, with the chemical formula SO3. It is a white crystalline solid that is highly reactive and readily combines with water to form sulfuric acid.
.
What Is SO3 Called?
SO3 is called sulfur trioxide.
.
What Is Sulfur Trioxide?
Sulfur trioxide is typically produced on an industrial scale by the oxidation of sulfur dioxide, which is a byproduct of many industrial processes.
.
It is used in the production of sulfuric acid, which is a key component in the production of fertilizers, detergents, and many other industrial and chemical products.
Sulfur trioxide is also a major air pollutant, as it is a component of acid rain and can contribute to respiratory problems in humans and other animals.
Therefore, the emissions of sulfur trioxide are regulated by environmental agencies in many countries.
.
Sulfur Trioxide Formula
The chemical formula of sulfur trioxide is SO3.
.
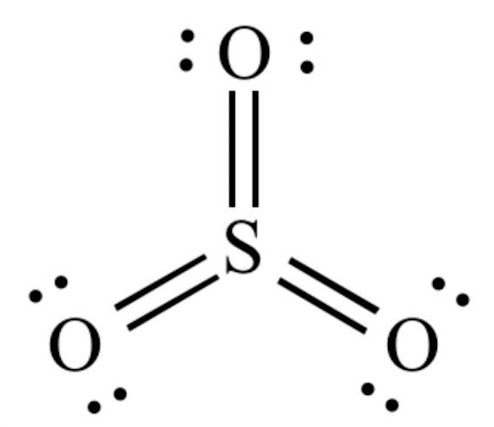
Sulfur Trioxide Shape
Sulfur trioxide (SO3) has a trigonal planar shape. The sulfur atom is located in the center, with three oxygen atoms arranged in a flat triangle around it, each at a bond angle of approximately 120 degrees.
The molecule has a net dipole moment, meaning it has a separation of charge between the sulfur and oxygen atoms, with the sulfur having a partial positive charge and the oxygen atoms having partial negative charges.
.
.
Sulfur Trioxide Polar or Nonpolar?
Sulfur trioxide (SO3) is a polar molecule. The sulfur atom in SO3 has a partial positive charge, while the three oxygen atoms have partial negative charges.
.
This is due to the arrangement of the atoms in the molecule, which results in an overall dipole moment.
The molecule is trigonal planar, with the three oxygen atoms located at the corners of an equilateral triangle around the central sulfur atom.
The bond dipoles of the sulfur-oxygen bonds do not cancel out, resulting in a net dipole moment and making SO3 a polar molecule.
.
What Is Sulfur Trioxide Used for?
Sulfur trioxide is primarily used in the production of sulfuric acid, which is a key component in the production of fertilizers, detergents, and many other industrial and chemical products.
.
Other uses of sulfur trioxide include:
| Desiccant | Sulfur trioxide can be used as a desiccant, meaning it can absorb moisture from the air. |
| Polymerization catalyst | Sulfur trioxide can be used as a catalyst in the polymerization of certain plastics, such as polyethylene terephthalate (PET). |
| In the production of other chemicals | Sulfur trioxide is used in the production of other chemicals, such as oleum, which is a mixture of sulfur trioxide and sulfuric acid used in the production of surfactants and detergents. |
| Manufacturing of dyes and pigments | Sulfur trioxide is used in the production of certain dyes and pigments, such as sulfur dyes. |
| Production of pharmaceuticals | Sulfur trioxide is used in the production of certain pharmaceuticals, such as antibiotics and analgesics. |
.
It’s important to note that sulfur trioxide is a highly reactive and potentially hazardous substance, and appropriate safety measures must be taken during its transportation, handling, and use.
.
Is SO3 a Harmful Gas?
What are the dangers of SO3?
Yes, SO3 (sulfur trioxide) is a harmful gas. It is a highly reactive and corrosive substance that can cause severe respiratory irritation and damage to the eyes, skin, and mucous membranes on contact. Inhalation of sulfur trioxide can cause acute respiratory distress, which can be fatal in severe cases.
In addition to its acute toxicity, sulfur trioxide is also a major air pollutant that contributes to the formation of acid rain, which can have harmful effects on plants, animals, and ecosystems.
Therefore, appropriate safety measures must be taken during the transportation, handling, and use of sulfur trioxide, and its emissions must be regulated to prevent environmental and health hazards.
