.

Sulfur can be dissolved in a variety of substances, like carbon disulfide, benzene, toluene, and some other nonpolar organic solvents.
.
It is also soluble in a few polar solvents, like sulfuric acid and nitric acid.
.
.
What Is the Best Way to Dissolve Sulfur?
The best way to dissolve sulfur depends on the purpose and also the context of the dissolution.
.
However, sulfur is most commonly dissolved in nonpolar organic solvents like carbon disulfide, benzene, or toluene, because they have a high affinity for sulfur due to the similar chemical properties of them.
It is also possible to dissolve sulfur in polar solvents like sulfuric acid and nitric acid, but these are usually less common and need more caution in handling due to the corrosive nature of these acids.
.
What is Polar and Nonpolar Solvent?
.
.
The process of dissolving sulfur in a solvent generally includes heating the mixture to a temperature above the melting point of sulfur, which is 115.2 °C. Once the sulfur is melted, it can be added to the solvent and stirred or agitated until it dissolves.
The solubility of sulfur in a particular solvent may vary depending on some factors such as temperature, pressure, and the concentration of the solvent.
It is worth noting that while sulfur can be dissolved in various solvents, it may not always be completely soluble.
In some cases, undissolved sulfur may settle at the bottom of the container or form a suspension. It is also significant to handle sulfur and its solvents with care, as they can be flammable, toxic, or hazardous to health if you do not use them properly.
.
What Is the Solubility of Sulfur?
The solubility of sulfur can vary depending on the factors below:
- temperature
- pressure
- the solvent being used
.
It is worth mentioning that at standard temperature and pressure (STP), which is 25 °C and 1 atmosphere (101.3 kPa), sulfur is insoluble in water and most polar solvents. However, it can be dissolved in nonpolar organic solvents including carbon disulfide, benzene, and toluene.
.
At higher temperatures and pressures, the solubility of sulfur in different solvents can vary.
For example, at the formation temperature of 98.9 °C and a pressure of 49.8 MPa, the solubility of sulfur in water reaches 0.968 g/m3.
This is relatively high.
As the pressure decreases to 15 MPa, the solubility of sulfur in water reduces to 0.031 g/m3, indicating that lower pressure decreases sulfur solubility.
At the formation pressure of 49.8 MPa, the solubility of sulfur in water drops to 0.076 g/m3 as the temperature decreases to 40 °C. The solubility of sulfur can also be affected by the presence of other substances or impurities in the solvent.
.

.
Typically, the solubility of sulfur is relatively low in most solvents, especially in water, making it a challenge to dissolve.
Therefore, nonpolar organic solvents such as carbon disulfide, benzene, and toluene are generally applied to dissolve sulfur due to their high solubility and affinity for sulfur.
.
Does Sulphur Dissolve in Hot Water?
.
Sulfur is generally considered insoluble in water. It is insoluble in hot water too. At room temperature and pressure, the solubility of sulfur in water is only about 0.003 g/L. This is extremely low!
.
The solubility of sulfur in water increases somewhat with temperature, but it still remains very low.
Therefore, sulfur is unlikely to dissolve significantly in water, even at high temperatures.
However, there are some cases where sulfur can react with water to form sulfuric acid, which is soluble in water.
When sulfur reacts with hot concentrated sulfuric acid, for example, it can form sulfates that are soluble in water.
However, this is a chemical reaction rather than simple dissolution. It involves the production of a new substance rather than the dissolution of the sulfur itself.
In general, nonpolar organic solvents such as carbon disulfide, benzene, and toluene are more effective in dissolving sulfur due to their similar chemical properties and high solubility.
.
Can Sulphur Dissolve in Oil?
.
Sulfur can dissolve in certain types of oil, depending on their chemical composition and properties.
.
For example, sulfur can dissolve in some organic oils like mineral oil, vegetable oil, and animal fat.
However, the solubility of sulfur in oil depends on different factors, such as the temperature, pressure, and concentration of the sulfur and oil.
In some cases, the presence of sulfur in oil can cause problems including corrosion and fouling of equipment, so it is important to carefully monitor and control the levels of sulfur in oil.
Additionally, the solubility of sulfur in oil can also depend on the specific type of sulfur compound, as some are more soluble than others.
.
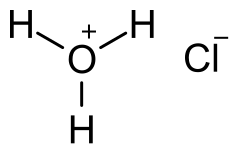
Does Sulphur Dissolve in Hydrochloric Acid?
.
Sulfur is insoluble in hydrochloric acid. Hydrochloric acid is a strong acid. It can dissolve many metals and other compounds, but sulfur is not one of them.
.
.
Sulfur is a non-metallic element that is not very reactive with acids.
When sulfur comes into contact with hydrochloric acid, it may react to produce hydrogen sulfide gas, but it will not dissolve.
However, if sulfur is first melted or burned, it can react with hydrochloric acid to produce hydrogen sulfide gas and sulfur dioxide gas. The reaction between sulfur and hydrochloric acid can be represented by the chemical equation:
S + 2 HCl → H2S + SO2 + Cl2
So, while sulfur does not dissolve in hydrochloric acid, it can react with it under certain conditions.
.
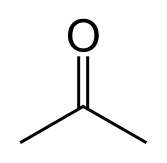
Does Sulphur Dissolve in Acetone?
.
Sulfur is not very soluble in acetone, but it can dissolve to some extent. Acetone is a polar aprotic solvent, which means that it has a dipole moment but does not contain any acidic or basic protons. Sulfur is a non-polar substance, so it is not expected to be very soluble in polar solvents like acetone.
.
.
However, sulfur can dissolve in acetone to some extent due to the polarizability of sulfur. When sulfur is added to acetone, some of the sulfur molecules dissolve in the solvent, while others remain undissolved. The solubility of sulfur in acetone increases with increasing temperature and with the addition of certain solubilizing agents like sodium sulfide.
Overall, the solubility of sulfur in acetone is relatively low, and other solvents like carbon disulfide or chloroform are typically used for dissolving larger amounts of sulfur.
.
Can Sulphur Dissolve in Kerosene?
.
Sulfur is not very soluble in kerosene, but it can dissolve to some extent. Kerosene is a nonpolar solvent, and sulfur is also a nonpolar substance, so the solubility of sulfur in kerosene is limited.
.
However, sulfur can dissolve in kerosene to some extent due to the van der Waals forces between the sulfur molecules and the kerosene molecules. When sulfur is added to kerosene, some of the sulfur molecules dissolve in the solvent, while others remain undissolved. The solubility of sulfur in kerosene increases with increasing temperature.
Overall, the solubility of sulfur in kerosene is relatively low, and other solvents like carbon disulfide or benzene are typically used for dissolving larger amounts of sulfur.
.
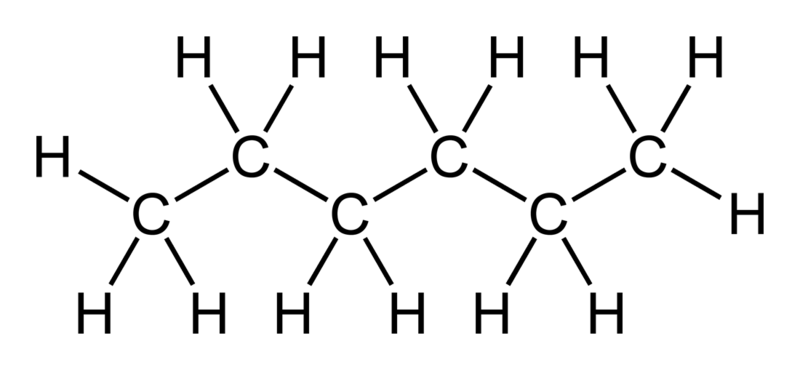
Does Sulfur Dissolve in Hexane?
.
Sulfur is not very soluble in hexane, but it can dissolve to some extent. Hexane is a nonpolar solvent, and sulfur is also a nonpolar substance, so the solubility of sulfur in hexane is limited.
.
.
However, sulfur can dissolve in hexane to some extent due to the van der Waals forces between the sulfur molecules and the hexane molecules. When sulfur is added to hexane, some of the sulfur molecules dissolve in the solvent, while others remain undissolved. The solubility of sulfur in hexane increases with increasing temperature.
Overall, the solubility of sulfur in hexane is relatively low, and other solvents like carbon disulfide or benzene are typically used for dissolving larger amounts of sulfur.
.
Is Sulfur Soluble in Xylene?
.
Sulfur is moderately soluble in xylene. Xylene is a nonpolar solvent, and sulfur is also a nonpolar substance. Xylene can dissolve a wide variety of organic compounds, including sulfur, due to the Van der Waals forces between the xylene molecules and the sulfur molecules.
.
.
The solubility of sulfur in xylene increases with increasing temperature. At room temperature, the solubility of sulfur in xylene is relatively low, but it increases as the temperature is raised.
However, the maximum solubility of sulfur in xylene is still relatively limited, and other solvents like carbon disulfide or benzene may be used for dissolving larger amounts of sulfur.
