.

What Is Sulfuric Acid?
.
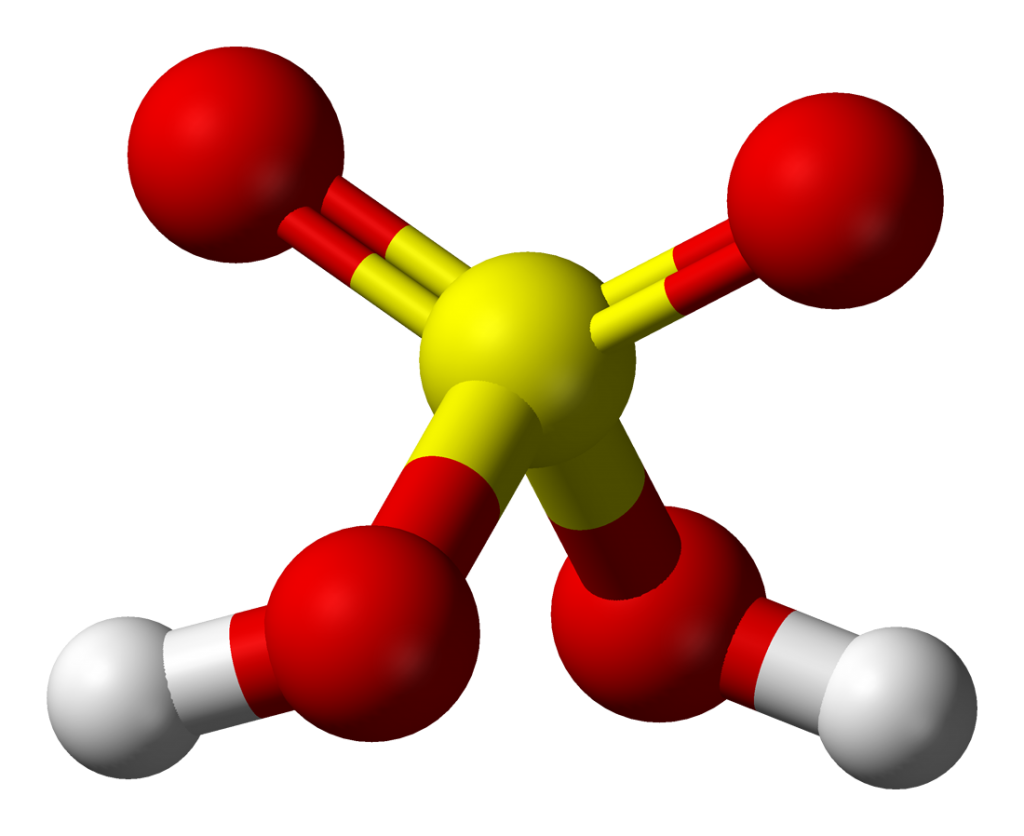
Sulfuric acid is a highly corrosive and strong mineral acid.
its molecular formula is H2SO4.
.
.
.
It is often referred to as the “king of chemicals” because it is one of the most important and widely used industrial chemicals.
.
Sulfuric acid is a dense, colorless, and odorless liquid that is soluble in water and has a boiling point of about 337 °C.
.
.
Sulfuric acid is produced in large quantities by the chemical industry and is used in a wide variety of applications, including:
.
- Production of fertilizers: Sulfuric acid is a key component in the production of phosphoric acid, which is used to make fertilizers.
- Chemical synthesis: Sulfuric acid is used as a catalyst in the production of a wide range of chemicals, including detergents, synthetic fibers, dyes, and pharmaceuticals.
- Petroleum refining: Sulfuric acid is used to remove impurities from crude oil and to make gasoline and other fuels.
- Battery manufacturing: Sulfuric acid is used in the production of lead-acid batteries, which are used in cars, trucks, and other vehicles.
- Metal processing: Sulfuric acid is used to leach metals such as copper, zinc, and aluminum from ores, and to remove rust and scale from metal surfaces.
.
Sulfuric acid is highly corrosive and can cause severe burns if it comes into contact with skin or eyes. It is also a potent oxidizing agent and can react violently with other chemicals, especially when heated.
Therefore, it should be handled with extreme care and stored in appropriate containers to prevent leaks and spills.
.
What Is The Main Ingredient of Sulfuric Acid?
The main ingredient of sulfuric acid is sulfur trioxide (SO3), which is produced by the combustion of sulfur dioxide (SO2) gas.
.
Sulfur dioxide is typically generated by burning sulfur or sulfide ores, such as iron sulfide (FeS2) or copper sulfide (CuS).
.

.
The reaction for the production of sulfur trioxide from sulfur dioxide is as follows:
2 SO2 (g) + O2 (g) → 2 SO3 (g)
.
Sulfur trioxide is then dissolved in water to produce sulfuric acid (H2SO4), which is the final product. The reaction for the production of sulfuric acid from sulfur trioxide is as follows:
SO3 (g) + H2O (l) → H2SO4 (aq)
.
How Is Sulfuric Acid Manufactured?
.

.
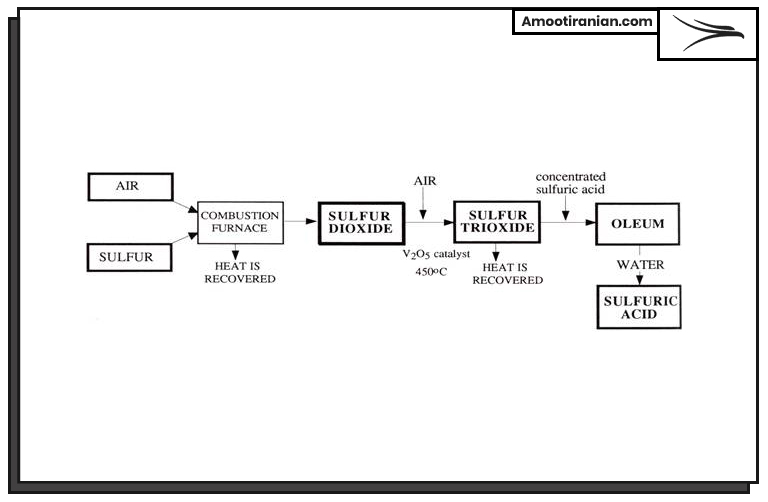
Sulfuric acid production process is as follow, each of these steps are explained fully in the next part.
.
- Sulfur melting and purification
- Sulfur burning
- Conversion of SO2 to SO3
- Absorption of SO3 in water
- Concentration of sulfuric acid
- Purification and storage
.
How to make sulfuric acid step by step?
The production of sulfuric acid from sulfur typically involves several stages, which can be summarized as follows:
.
1_Sulfur Melting And Purification
The first step in sulfuric acid production process involves melting and purifying the sulfur to remove any impurities that could affect the quality of the final product. This procedure has the following steps:
- Melting the sulfur
- Filtering the sulfur
- Purifying the sulfur
.
First, sulfur is heated to a temperature of about 120-160 °C. At this temperature, the sulfur becomes a liquid and can be easily handled and transported.
During the melting process, care must be taken to ensure that the sulfur is heated evenly and that it does not overheat or burn. This can be achieved by using special equipment such as heating coils or by carefully controlling the temperature of the furnace.
.
Second, the melted sulfur is usually passed through a filtration system to remove any impurities or solid particles that may be present. This helps to ensure that the sulfur is of high purity and will produce a high-quality sulfuric acid.
The filtration system may consist of a series of screens or mesh filters that trap any solid particles, or it may use other types of filters such as activated carbon or ion exchange resins to remove impurities
.
Third, after the sulfur has been filtered, it may undergo further purification to remove any remaining impurities. This can be done by a variety of methods, including distillation, fractional crystallization, or chemical treatment.
.
Distillation involves heating the sulfur to a high temperature and collecting the purified liquid sulfur that is produced. Fractional crystallization involves cooling the sulfur to a low temperature and allowing it to crystallize, with the pure sulfur crystals being separated from the impurities.
Chemical treatment may involve adding chemicals such as hydrogen peroxide or chlorine to the melted sulfur to react with and remove impurities.
.
Overall, the melting and purification of sulfur is a crucial step in the production of high-quality sulfuric acid, as it ensures that the sulfur is free from impurities and will produce a pure and high-quality final product.
.

.
2_Sulfur Burning
Once the sulfur is purified, it is burned in a furnace to produce sulfur dioxide (SO2) gas, according to the following reaction:
S (s) + O2 (g) → SO2 (g)
.
The combustion process usually takes place in a multiple hearth furnace or a fluidized bed reactor.
A multiple hearth furnace and a fluidized bed reactor are two types of industrial furnaces used for different applications. Here is a brief explanation of each:
_____Multiple hearth furnace: A multiple hearth furnace is a type of industrial furnace that is commonly used for the combustion of solid materials, including sulfur. The furnace consists of a series of circular hearths stacked on top of each other, with the sulfur being burned on each hearth as it rotates through the furnace.
As the sulfur burns, it releases heat, which is absorbed by the refractory lining of the furnace. The rotation of the hearths helps to ensure that the sulfur is evenly burned and that the combustion process is efficient. Multiple hearth furnaces are often used in the production of sulfuric acid, as well as for the incineration of other solid wastes.
_____Fluidized bed reactor: A fluidized bed reactor is a type of industrial reactor that is used for a variety of applications, including the combustion of solid materials such as coal or biomass. In a fluidized bed reactor, a bed of solid particles is suspended in a stream of gas, usually air.
As the gas flows through the bed, it causes the particles to become fluidized, or suspended in the gas stream. The fluidized particles provide a large surface area for combustion to occur, which results in a more efficient and complete combustion process.
Fluidized bed reactors are often used in the production of sulfuric acid from sulfur dioxide gas, as well as for other applications such as the gasification of coal or the production of hydrogen from natural gas.
.
3_Conversion Of SO2 to SO3
In the next step, the sulfur dioxide gas is converted to sulfur trioxide (SO3), which is the precursor to sulfuric acid. This is typically done using a catalyst such as vanadium pentoxide (V2O5), according to the following reaction:
SO2 (g) + O2 (g) → 2 SO3 (g)
Vanadium pentoxide (V2O5) is a chemical compound that consists of two atoms of vanadium and five atoms of oxygen. It is a yellow-orange or brownish powder that is highly toxic and reactive.
Vanadium pentoxide is widely used as a catalyst in the production of sulfuric acid from sulfur dioxide gas. It helps to convert the sulfur dioxide gas into sulfur trioxide, which is then reacted with water to produce sulfuric acid.
The addition of vanadium pentoxide to the reaction helps to increase the efficiency of the conversion process and to reduce the amount of sulfuric acid byproducts that are produced.
.
4_Absorption of SO3 in Water
The sulfur trioxide gas is then absorbed in water to form sulfuric acid. This step is highly exothermic and releases a large amount of heat, which can be used to generate steam or to heat other parts of the process. The reaction is typically carried out in a tower called an absorption tower or contact tower.
SO3 (g) + H2O (l) → H2SO4 (aq)
An absorption tower, also known as a contact tower, is a key component in the production of sulfuric acid.
The absorption tower is typically a tall, vertical vessel that contains a series of trays or packing material. The sulfur dioxide gas is fed into the bottom of the tower, while a stream of concentrated sulfuric acid is fed in from the top. As the gas rises through the tower, it comes into contact with the sulfuric acid, which acts as a catalyst to promote the conversion of SO2 to H2SO4.
The packing material or trays inside the tower serve to increase the surface area of the sulfuric acid, allowing more efficient contact with the sulfur dioxide gas. The tower is typically operated at a high temperature and pressure, which further enhances the efficiency of the conversion process.
.
5_Concentration of Sulfuric Acid
The sulfuric acid solution produced in the absorption tower is usually diluted, and then concentrated using a series of evaporation and condensation steps.
This process is typically carried out in a series of vessels called concentrators, where the sulfuric acid solution is heated to evaporate water, and then cooled to condense the concentrated sulfuric acid.
After the sulfuric acid is produced in the absorption tower, it is typically in a dilute form, with a concentration of around 50-70% while the concentrated sulfuric acid solution produced in this process typically has a concentration of around 98-99%.
The first step in this process is typically carried out in a vessel called a concentrator or an evaporator. The dilute sulfuric acid solution is heated under vacuum conditions, which helps to reduce the boiling point of the solution and to prevent any decomposition of the acid. As the solution is heated, water begins to evaporate, leaving behind a more concentrated sulfuric acid solution.
The concentrated sulfuric acid solution is then transferred to another vessel for further processing. This process is repeated in a series of concentrators, with each stage producing a more concentrated sulfuric acid solution.
In order to achieve the desired concentration of sulfuric acid, the solution is typically subjected to a final step of condensation.
In this step, the concentrated sulfuric acid solution is cooled, causing the water vapor to condense and leaving behind a highly concentrated sulfuric acid solution.
.
.
6_Purification and storage
The final step in sulfuric acid production process involves purifying and storing the concentrated acid. This may involve further treatment to remove impurities such as heavy metals or organic compounds, and then storing the acid in tanks or drums for shipment to customers.
This purification step can involve a range of techniques, such as filtration, ion exchange, or solvent extraction, depending on the specific impurities present.
One common method of purification is to treat the sulfuric acid with activated carbon, which can absorb a wide range of impurities, such as heavy metals or organic compounds. The activated carbon is typically added to the acid in a separate vessel and allowed to soak for a period of time before being filtered out.
.

.
Once the sulfuric acid has been purified, it is typically stored in large tanks or drums for shipment to customers.
The storage tanks must be constructed of materials that are resistant to the corrosive nature of sulfuric acid, such as stainless steel or lined with a special coating.
During storage, the sulfuric acid must be kept at a controlled temperature to prevent it from freezing or boiling, which could cause damage to the storage tanks or drums.
In addition, it must be stored away from other chemicals that could react with it, and away from sources of heat or ignition, to prevent the risk of fire or explosion.
When the sulfuric acid is ready to be shipped to customers, it is typically loaded into tanker trucks, railcars, or shipping containers for transport.
During transportation, the acid must be secured and protected from damage, and all applicable safety regulations must be followed to ensure the safe handling and transport of the acid.
Overall, the sulfuric acid production process from sulfur is a complex process that involves several stages of chemical reactions, heat transfer, and material handling.
It requires careful control and monitoring to ensure high quality and safety, and is typically carried out in large industrial plants.
