What is sulfur good for? What is sulfur used for in everyday life? What is sulfur used for in fertilizer? Continue reading this page to understand what is sulfur used for in the modern world and so many other useful data about sulfur.
What is sulfur?

Sulfur is a chemical element with the symbol S and atomic number 16. It is a nonmetal and lies in the third row of the periodic table.
Sulfur is a yellow, brittle, odorless solid at room temperature. It is insoluble in water but dissolves in many organic solvents. Sulfur has several allotropes, or different physical forms, including rhombic sulfur and monoclinic sulfur.
Rhombic sulfur is the most stable and commonly found form of sulfur at room temperature and is used in a variety of industrial processes.
Is Sulphur easily available?
- Sulfur is abundant in the earth’s crust and is found in many minerals, including gypsum, Epsom salt, and pyrite.
- It is also present in natural gas, crude oil, and coal.

Sulfur is an essential element for life. It is found in amino acids and proteins, including the essential amino acid methionine. Sulfur is also an important component of vitamins, such as biotin and thiamine.
Sulfur has a long history of use in human culture. It has been used for centuries in the production of gunpowder and in the treatment of various skin diseases. Sulfur was also used in ancient times for fumigation and in the preservation of food. In the Middle Ages, sulfur was used as a treatment for the plague.
Despite its many uses, sulfur can also be harmful to human health and the environment. Exposure to high levels of sulfur dioxide can cause respiratory problems and can contribute to acid rain.
Sulfur dioxide can also contribute to the formation of particulate matter in the air, which can have negative impacts on human health. Sulfur can also be toxic to some organisms, including plants and animals.
If you want to find out what is sulfur used for, continue reading this page.
How Does Sulfur Work in Various Industries?
1_Sulfur is used to produce sulfuric acid
Sulfuric acid is one of the most important industrial chemicals, with an annual global production of around 180 million metric tons. The majority of sulfuric acid is produced by the contact process, which involves the oxidation of sulfur dioxide to sulfur trioxide, followed by the reaction of sulfur trioxide with water to form sulfuric acid.
The primary source of sulfur dioxide for this process is elemental sulfur, which is burned to produce sulfur dioxide gas.
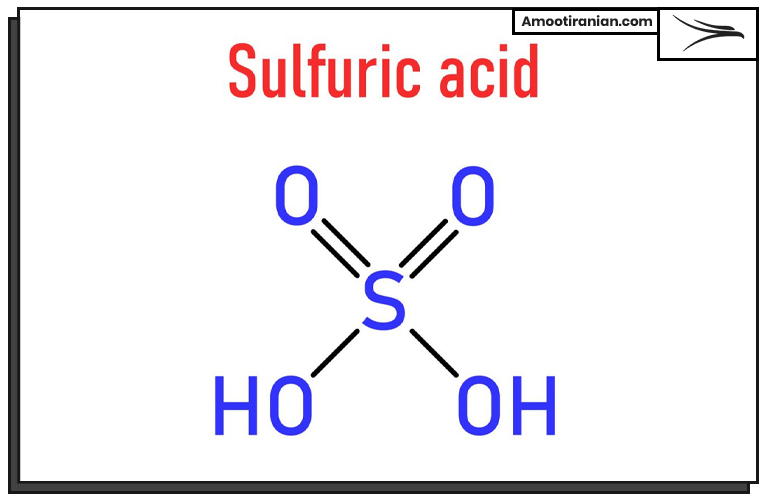
The production of sulfuric acid begins with the combustion of sulfur, which results in the formation of sulfur dioxide gas. The sulfur dioxide is then purified and dried before being introduced into a reaction chamber, where it is oxidized to sulfur trioxide using a catalyst such as vanadium pentoxide.
This reaction is highly exothermic and releases a large amount of heat, which must be carefully controlled to prevent damage to the equipment.
Once the sulfur dioxide has been converted to sulfur trioxide, it is mixed with a stream of air and passed over a catalyst bed, typically made of silica, to facilitate the formation of sulfuric acid.
The sulfur trioxide is then cooled and condensed to form concentrated sulfuric acid. The final product is typically between 93-98% sulfuric acid, with the remaining percentage consisting of water and other impurities.
The contact process is highly efficient, and the majority of the sulfur dioxide is converted to sulfuric acid.
However, the process does produce significant amounts of nitrogen oxides and other pollutants, which can have negative environmental impacts.
As a result, modern sulfuric acid production facilities are equipped with extensive pollution control measures, including scrubbers and electrostatic precipitators, to remove these pollutants before they are released into the atmosphere.
Now, we continue with various uses of sulfuric acid in different industries, which are all the final product of sulfur:
2_Chmical fertilizers
What is sulfur used for in fertilizer industry?
After producing sulfuric acid from sulfur, sulfuric acid is used to make chemical fertilizers for crops’ nutrients.
Sulfuric acid has a wide range of industrial applications and is used in the production of a variety of chemicals, including fertilizers.

The use of sulfuric acid in the production of fertilizers is one of its most important applications. Sulfuric acid is used to convert phosphate rock into soluble forms of phosphate, which can be taken up by plants.
This process, known as the “wet process,” is used to produce approximately 90% of the world’s phosphate fertilizers. Sulfuric acid is also used in the production of ammonium sulfate, which is another important fertilizer.
What are examples of sulfur fertilizer?
- Fertilizers containing sulphate
- Fertilizers containing elemental sulfur
- Fertilizers containing a combination of sulphate and elemental sulfur
- Liquid sulfur fertilizer
3_Dyes
Sulfur can be used to produce dyes through a process called sulfuration.
In this process, the sulfur is first melted and then combined with an organic compound such as aniline, phenol, or naphthalene, which acts as a chromophore, providing the color to the dye.
The mixture of the sulfur and the organic compound is then heated at high temperature and under pressure in the presence of a catalyst, such as iron or aluminum oxide.
This causes the sulfur to react with the organic compound, leading to the formation of the dye.

The specific conditions of the sulfuration process, including temperature, pressure, and the choice of organic compound and catalyst, can all affect the color and properties of the resulting dye.
Sulfur dyes are commonly used to produce colors such as black, brown, and gray, as well as darker shades of blue, green, and red. They are known for their excellent lightfastness and washfastness, making them popular for use in textiles and other applications where colorfastness is important.
3_Detergents
Sulfur is not directly used to produce detergents, but sulfur compounds are commonly used in the production of various types of detergents.
One common sulfur compound used in detergent production is sodium dodecyl sulfate (SDS), also known as sodium lauryl sulfate.
SDS is a surfactant that is used to help remove dirt and oil from surfaces.
In the production of SDS, sulfuric acid is reacted with lauryl alcohol to form the sodium salt of lauryl sulfate. The resulting SDS is then purified and dried before being used in detergents.
Sulfur compounds are also used in the production of other types of detergents, such as alkylbenzene sulfonates (ABS).
ABS is made by reacting an alkylbenzene with sulfur trioxide to form an intermediate compound called an alkylbenzenesulfonic acid.
The alkylbenzenesulfonic acid is then neutralized with a base, such as sodium hydroxide, to form the final product.
Sulfur compounds are important in detergent production because they help to reduce the surface tension of water, allowing the detergent to more effectively penetrate and remove dirt and oil from surfaces. Additionally, sulfur compounds can help to stabilize the foam produced by detergents, which can aid in the cleaning process.
4_pharmaceuticals

Sulfur and its compounds are used in various ways in the production of pharmaceuticals. Some common uses of sulfur in pharmaceuticals include:
- Production of antibiotics: Sulfur compounds, such as sulfonamides, are used as a key component in the production of antibiotics. These compounds work by inhibiting the growth of bacteria, making them useful for treating bacterial infections.
- Production of anti-inflammatory drugs: Sulfur is a key component in the production of some anti-inflammatory drugs, such as ibuprofen and naproxen. These drugs work by reducing inflammation and pain in the body.
- Production of anti-cancer drugs: Sulfur compounds, such as thiols, are used in the production of some anti-cancer drugs. These compounds work by interfering with the growth and division of cancer cells, making them useful for treating some types of cancer.
- Production of topical treatments: Sulfur is also used in the production of some topical treatments for skin conditions such as acne and rosacea. Sulfur can help to reduce inflammation and improve the appearance of the skin.
- Production of diagnostic agents: Sulfur compounds are used in the production of some diagnostic agents, such as radioisotopes. These agents can be used to help diagnose and monitor certain medical conditions.
In each of these applications, sulfur is typically used as a starting material or as a key component in the synthesis of the final product.
The specific properties and reactivity of sulfur and its compounds make them useful for a wide range of pharmaceutical applications.
5_Metal (mostly zinc and copper) refining
Sulfur is commonly used in the refining of metals such as zinc and copper. One of the main uses of sulfur in metal refining is as a reducing agent, which can help to remove impurities from the metal.
In the case of zinc refining, sulfur dioxide (SO2) is typically used as a reducing agent. This involves roasting zinc sulfide (ZnS) ore in the presence of air, which oxidizes the sulfur in the ore to SO2 gas.
The SO2 gas is then reacted with the zinc oxide (ZnO) that is also present in the ore, producing zinc metal and sulfur dioxide. The sulfur dioxide gas can then be converted into sulfuric acid or used in other industrial processes.
In copper refining, sulfur is used to remove impurities such as oxygen, iron, and other metals. One common method for refining copper involves smelting the copper ore with sulfur, which reacts with the oxygen and other impurities to form sulfur dioxide gas.

The sulfur dioxide gas is then captured and converted into sulfuric acid or used in other industrial processes. The resulting copper is then further refined through electrolysis or other methods to produce high-purity copper for use in various applications.
Sulfur can also be used in the production of copper alloys, such as brass and bronze. In these applications, sulfur is typically added in small amounts to improve the machinability of the alloy and to help control the grain size and distribution.
Overall, sulfur plays an important role in the refining of metals such as zinc and copper, helping to remove impurities and produce high-quality metal products.
6_batteries
Sulfur is used in the production of batteries, specifically in a type of battery known as a lithium-sulfur (Li-S) battery. Li-S batteries are a promising alternative to the more commonly used lithium-ion (Li-ion) batteries, as they offer higher energy density and lower cost.
In a Li-S battery, sulfur is used as the cathode material, which is the electrode that accepts electrons during discharge. The anode is typically made of lithium metal or another lithium-containing material.
During discharge, lithium ions from the anode move through the electrolyte and react with the sulfur in the cathode to form lithium sulfide (Li2S).

This reaction releases electrons, which flow through an external circuit and can be used to power a device.
During charging, the process is reversed, with lithium ions moving back to the anode and sulfur being re-deposited on the cathode.
One of the key advantages of Li-S batteries is their high theoretical energy density, which is due to the high energy content of sulfur.
However, there are also some challenges associated with the use of sulfur in batteries, such as the low conductivity of sulfur and the tendency for the sulfur to dissolve into the electrolyte over time.
To address these challenges, researchers are exploring various strategies for improving the performance of Li-S batteries, such as using different cathode materials, modifying the electrolyte, and developing new manufacturing techniques.
With further research and development, Li-S batteries have the potential to become an important technology for energy storage in a wide range of applications.
7_Explosives
Which acid is used in explosive?
Sulfur is a key ingredient in the production of explosives, particularly black powder and gunpowder. Black powder is a simple explosive mixture that has been used for centuries, while gunpowder is a more refined version of black powder that is used in modern firearms.
In black powder, sulfur is used as a fuel and a reducing agent, which helps to release the energy stored in the other ingredients of the mixture.
The other main ingredients of black powder are charcoal and potassium nitrate (saltpeter). The mixture is typically prepared by grinding the ingredients together and then forming them into pellets or grains.
In gunpowder, sulfur is used as a fuel and a stabilizer. The other main ingredients of gunpowder are saltpeter and charcoal, which provide the oxidizing and reducing agents, respectively.

The mixture is typically prepared by carefully measuring and mixing the ingredients together, then grinding the mixture into a fine powder.
The role of sulfur in these explosives is to provide additional fuel and to help regulate the rate of combustion.
Sulfur is a low explosive on its own, meaning that it burns relatively slowly and steadily, rather than exploding quickly. When combined with other ingredients, such as saltpeter and charcoal, the sulfur can help to control the rate of combustion and produce a more controlled explosion.
Overall, sulfur plays an important role in the production of explosives, particularly black powder and gunpowder. While these explosives are relatively simple compared to modern explosives, they are still used in some traditional applications, such as in fireworks and historical reenactments.
7_Synthetic fibers, such as nylon and polyester
What is sulfur used for in synthetic fibers?
Sulfur is used in the production of synthetic fibers such as nylon and polyester as a catalyst and a component of the fiber-forming process.
Nylon is a synthetic polyamide fiber that is produced through the reaction of diamines and dicarboxylic acids.
Sulfuric acid is often used as a catalyst in this reaction to speed up the formation of the polymer chains. The resulting nylon polymer can then be extruded into fibers and further processed into yarns and fabrics.
Polyester is a synthetic polymer that is produced through the reaction of dicarboxylic acids and diols.

Sulfuric acid is also commonly used as a catalyst in this reaction, although other catalysts such as phosphoric acid or zinc acetate can also be used. The resulting polyester polymer can then be extruded into fibers and further processed into yarns and fabrics.
In addition to being used as a catalyst in the production of synthetic fibers, sulfur is also used as a component of the spinning solution that is used to form the fibers.
For example, in the production of rayon, a synthetic fiber made from cellulose, sulfuric acid is used to dissolve the cellulose and create a spinning solution that can be extruded into fibers.
Overall, sulfur plays an important role in the production of synthetic fibers such as nylon and polyester, helping to catalyze the polymerization reaction and form the fibers into usable materials.
8_Wood preservatives
Sulfuric acid can be used in the production of wood preservatives as a key component in the production of the active ingredient. The most common type of wood preservative that uses sulfuric acid is called acid copper chromate (ACC).
The production of ACC typically involves dissolving copper carbonate and sodium dichromate in a mixture of water and sulfuric acid.
The resulting solution is then applied to the wood, where the copper and chromium ions penetrate the wood fibers and protect the wood against decay and insect damage.
Sulfuric acid is used in this process as a catalyst and a reactant. It helps to facilitate the reaction between the copper carbonate and the sodium dichromate, which produces the copper and chromium ions that are the active ingredients in the wood preservative.

While ACC is an effective wood preservative, it has some environmental concerns and health risks associated with it.
The use of chromium in wood preservatives has been linked to a range of health problems, and the Environmental Protection Agency (EPA) has restricted the use of certain types of wood preservatives containing chromium.
As a result, many wood preservatives have moved away from using sulfuric acid and chromate-based compounds in favor of other types of wood preservatives that are considered to be safer and more environmentally friendly.
These alternatives include borate-based wood preservatives and copper-based wood preservatives that use copper oxide or copper naphthenate as the active ingredient.
9_ Adhesives
What is sulfur used for? Does it have any use in the production of adhesives?
Sulfur is used to make sulfuric acid and Sulfuric acid may be used in the production of certain types of adhesives as a catalyst.
For example, sulfuric acid can be used as a catalyst in the production of polyvinyl acetate (PVA) adhesives, which are a type of water-based adhesive commonly used in woodworking and other applications. In this process, vinyl acetate monomers are polymerized using a free radical initiator and sulfuric acid as a catalyst.
The sulfuric acid helps to facilitate the polymerization reaction by accelerating the rate of the reaction and promoting the formation of the polymer chains. Once the polymerization is complete, the resulting PVA adhesive can be used to bond a wide range of materials, including wood, paper, and fabric.

It’s worth noting that while sulfuric acid is an important component in the production of PVA adhesives, it is used in very small quantities and is typically neutralized or removed from the final product. The use of sulfuric acid in this process is carefully controlled and regulated to ensure the safety of workers and to minimize the impact on the environment.
Overall, while sulfuric acid may be used as a catalyst in the production of certain types of adhesives, it is just one component in a complex process, and its use is carefully monitored and controlled to ensure the safety and quality of the final product.
10_Inorganic salts
Sulfuric acid is widely used in the production of inorganic salts, which are a class of chemical compounds that are formed by the reaction of an acid with a base. The reaction between sulfuric acid and a base, such as a metal oxide, hydroxide, or carbonate, can produce a wide range of inorganic salts.
The specific process for making inorganic salts using sulfuric acid can vary depending on the specific salt being produced.
In general, the process involves mixing the appropriate amount of sulfuric acid with the base in a reaction vessel, which causes a chemical reaction to occur.
The resulting salt is then typically filtered or evaporated to remove any remaining water or other impurities.
Some common examples of inorganic salts that are produced using sulfuric acid include:
- Copper sulfate: This blue crystalline salt is commonly used as a fungicide, herbicide, and pesticide. It is produced by reacting copper oxide or copper carbonate with sulfuric acid.
- Zinc sulfate: This colorless salt is used in a wide range of applications, including as a dietary supplement and in the production of rayon. It is produced by reacting zinc oxide or zinc carbonate with sulfuric acid.
- Ammonium sulfate: This white crystalline salt is used as a fertilizer, a flame retardant, and in the production of textiles. It is produced by reacting ammonia with sulfuric acid.
In addition to these salts, sulfuric acid is used in the production of a wide range of other inorganic compounds, including phosphates, nitrates, and sulfates.
Its ability to react with a wide range of bases and produce a wide range of products makes it a versatile and important chemical in the manufacturing industry.
What are some signs and symptoms from a brief exposure to sulfur?
Brief exposure to sulfur can cause a range of signs and symptoms, depending on the specific form of sulfur and the route of exposure. Here are some of the common signs and symptoms of brief exposure to sulfur:
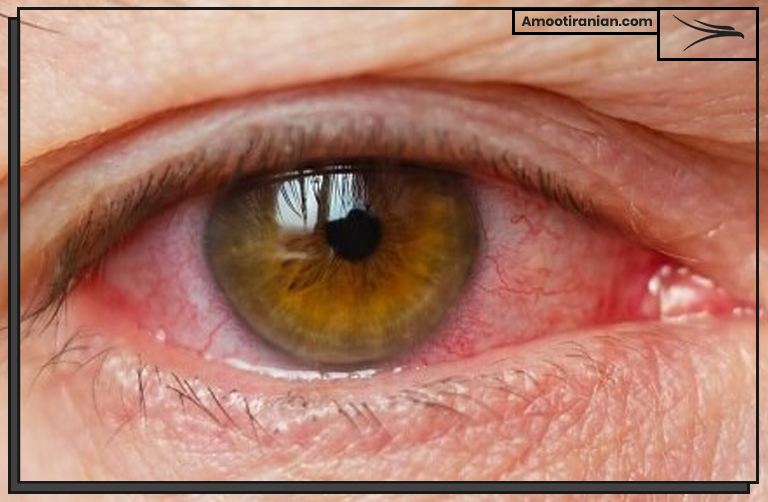
1_Skin and eye irritation: Exposure to sulfur compounds can cause skin and eye irritation, which may include redness, itching, burning, and swelling.
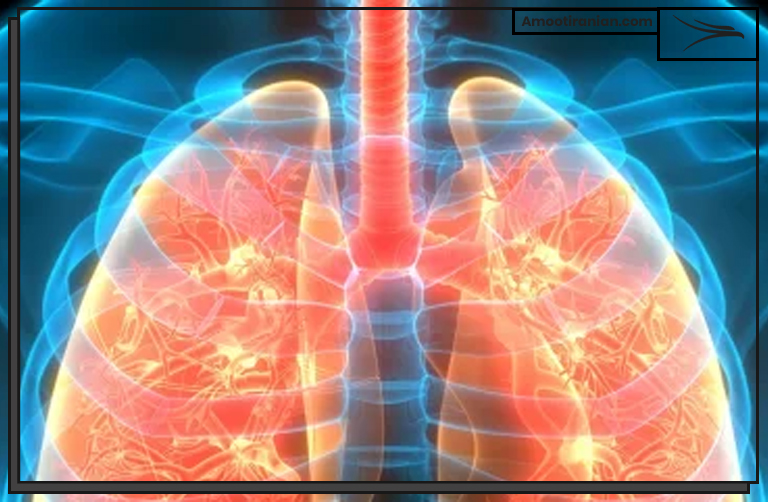
2_Respiratory irritation: Inhaling sulfur dioxide gas can cause irritation of the respiratory tract, including the nose, throat, and lungs. Symptoms may include coughing, wheezing, and difficulty breathing.
3_Headache: Exposure to sulfur compounds can also cause headaches, which may be mild to severe in intensity.

4_Nausea and vomiting: Ingesting or inhaling high levels of sulfur compounds can cause nausea and vomiting.

5_Fatigue and weakness: Brief exposure to sulfur can also cause fatigue, weakness, and general malaise.

6_Dizziness and confusion: High levels of exposure to sulfur can cause dizziness and confusion, which may impair a person’s ability to function normally.

7_Metal taste in the mouth: Ingesting high levels of sulfur can cause a metallic taste in the mouth.
It’s worth noting that the severity of the symptoms can vary depending on the concentration of sulfur and the duration of exposure.
If you suspect that you have been exposed to sulfur and are experiencing any of these symptoms, it is important to seek medical attention.
What happens to sulfur when it enters the body?
When sulfur enters the body, it can be metabolized and utilized by the body for various functions, but it can also be toxic in certain forms and at high levels of exposure.
In its elemental form, sulfur is not typically absorbed by the body and is generally considered to be non-toxic.
However, some sulfur-containing compounds, such as hydrogen sulfide gas, sulfur dioxide gas, and sulfites, can be toxic when inhaled or ingested in high concentrations.
When sulfur-containing compounds are absorbed into the body, they can be metabolized by enzymes and broken down into various forms.
Sulfur is an essential element in the human body and is a component of many amino acids, which are the building blocks of proteins. Sulfur is also important for the synthesis of several vitamins, such as biotin and thiamine, and plays a role in the metabolism of certain drugs.
However, exposure to high levels of certain sulfur-containing compounds can be toxic and cause a range of health problems.
For example, hydrogen sulfide gas can cause respiratory failure, seizures, and death at high concentrations, while sulfur dioxide gas can cause respiratory irritation, coughing, and difficulty breathing.
Sulfites can cause allergic reactions in some people, including hives, asthma, and anaphylaxis.
Overall, the effects of sulfur in the body depend on the specific form and concentration of sulfur, as well as the duration of exposure.
While some forms of sulfur are beneficial and even necessary for human health, others can be toxic and cause a range of health problems.

Summary
In conclusion, sulfur is a highly versatile and important element with a wide range of industrial and commercial uses. From the production of fertilizers, plastics, and detergents to the refining of metals, the production of pharmaceuticals, and the manufacturing of various chemicals and materials, sulfur is a crucial component in many industries.
Sulfuric acid, which is produced from sulfur, is a particularly important and widely used chemical, serving as a key component in the production of many other chemicals and materials.
Despite its many industrial applications, sulfur can also be found in natural sources such as volcanoes, hot springs, and certain minerals.
While sulfur and its various applications have brought many benefits to society, it is important to remember that sulfur and its compounds can also have potential negative effects on human health and the environment.
As such, it is crucial to use sulfur and its derivatives responsibly and with appropriate safety measures in place.
Overall, sulfur’s widespread use in a variety of industries highlights its importance in modern life and underscores the need for continued research and development in the field of sulfur chemistry.
